Chem 161 Chapter 9 Molecular Geometry Worksheet - Shape Determines Function Page 2
ADVERTISEMENT
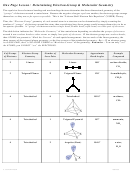
Molecule #2:
borane, BH
3
a. Draw the Lewis structure below.
b. 3D shape showing approximate bond angles
Lewis structure
B
c. Note that the three outer atoms make a triangle and all four atoms are in the
same plane, so this shape is called trigonal planar, where the bond angles are ________.
Molecule #3:
methane, CH
4
a. Draw the Lewis structure below.
b. 3D shape showing bond angles are 109.5˚.
Lewis structure
C
Thus, this shape is called tetrahedral,
in which the bond angles are 109.5˚.
Note: In tetrahedral, connecting the four outer atoms make four sides or faces.
(In Greek: “tetra” = four, “hedra” = face.)
CHEM
1 61:
C hapter
9
page
2
o f
4 0
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40